Climate Heresy: To Avoid Extinction We Need More, Not Less CO2
/A recent preprint advances the heretical idea that all life on Earth will perish in as little as 42,000 years unless we take action to boost – not lower – the CO2 level in the atmosphere. The preprint’s author claims that is when the level could fall to a critical 150 ppm (parts per million), below which plants die due to CO2 starvation.
Some of the arguments of author Brendan Godwin, a former Australian meteorologist, are sound. But Godwin seriously underestimates the time frame for possible extinction. It can easily be shown that the interval is in fact millions of years.
Plants are essential for life because they are the source, either directly or indirectly, of all the food that living creatures eat. Both CO2 and water, as well as sunlight, are necessary for the photosynthesis process by which plants grow. In the carbon cycle, the ultimate repository for CO2 pulled out of both the air and the oceans is limestone or calcium carbonate (CaCO3), of which there are two types: chemical and biological.
Chemical limestone is formed from the weathering over time of silicate rocks, which make up about 90% of the earth’s crust, and to a lesser extent, of carbonate rocks. Silicate weathering draws CO2 out of the atmosphere when the CO2 combines with rainwater to form carbonic acid (H2CO3) that dissolves silicates. A representative chemical reaction for calcium silicate (CaSiO3) is
CaSiO3 + 2CO2 + H2O → Ca2+ + 2HCO3- + SiO2.
The resulting calcium (Ca2+) and bicarbonate (HCO3-) ions, together with dissolved silica (SiO2), are then carried away mostly by rivers to the oceans. There, calcium carbonate (CaCO3) precipitates when marine organisms utilize the Ca2+ and HCO3- ions to build their skeletons and shells:
Ca2+ + 2HCO3- → CaCO3 + CO2 + H2O.
Once the organisms die, the CaCO3 skeletons and shells sink to the ocean floor and are deposited as chemical limestone in deep-sea sediment.
Biological limestone, on the other hand, comes from fossilized coral reefs and is approximately twice as abundant as chemical limestone. Just like the marine organisms or plankton that ultimately form chemical limestone, the polyps that constitute a coral build the chambers in which they live out of CaCO3. Biological limestone from accumulated coralline debris accumulates mainly in shallow ocean waters, and is transformed over time by plate tectonic processes into major outcrops on land and in the highest mountains – even the top of Mount Everest.
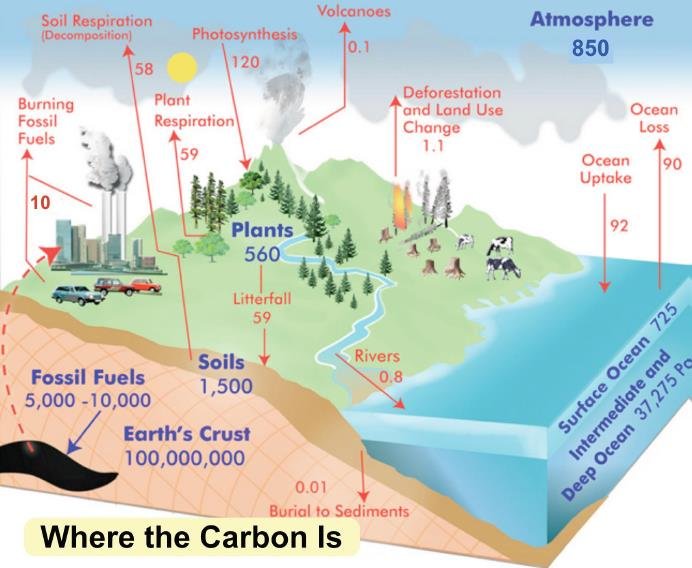
Godwin’s estimate of only 42,000 years before life is extinct stems from a misunderstanding about the carbon cycle, which is illustrated in the figure below depicting the global carbon budget in gigatonnes of carbon. Carbon stocks are shown in blue, with annual flows between carbon reservoirs shown in red.
The carbon sequestered as chemical limestone in deep-sea sediment, and as biological limestone, is represented by the 100 million gigatonnes stored in the earth’s crust. As you can see, today’s atmosphere contains approximately 850 gigatonnes of carbon (as CO2) and the oceans another 38,000 gigatonnes, most of which was originally dissolved as atmospheric CO2.
The erroneous estimate of Godwin simply divides the 38,000 gigatonnes of carbon in the oceans by 0.9 gigatonnes per year, which is the known rate of carbon sequestration into chemical and biological limestone combined; chemical weathering of silicate rocks contributes 0.3 gigatonnes per year, while fossilized coral contributes 0.6 gigatonnes per year.
This calculation is wrong because Godwin fails to understand that the carbon cycle is dynamic, with carbon constantly being exchanged between land, atmospheric and ocean reservoirs. The carbon sequestered into chemical and biological limestone is included in the flow from rivers to ocean and in ocean uptake in the figure above. But there are many flows in the opposite direction that replenish carbon in the atmosphere, even when fossil fuel burning is ignored. Simply depleting the ocean reservoir will not lead to extinction.
A realistic estimate can be made by assuming that atmospheric carbon will continue to decline at the same rate as it has over the past 540 million years. As shown in the next figure, the concentration of CO2 in the atmosphere over that period has dropped from a high of about 7,000 ppm at the beginning of the so-called Cambrian Explosion, to today’s 417 ppm.
Using a conversion factor of 2.13 gigatonnes of carbon per ppm of atmospheric CO2, the drop corresponds to an average decline of approximately 26 kilotonnes of carbon per year. At that rate, the 150 ppm (320 gigatonnes) level at which life on earth would begin to die will not be reached until 22 million years from now.
Given that the present CO2 level is rising due to fossil fuel emissions, the 22 million years is likely to be an underestimate. However, ecologist Patrick Moore points out that a future cessation of fossil fuel burning could make the next ice age – which may be only thousands of years away – devastating for humanity, as temperatures and CO2 levels could fall to unprecedentedly low levels, drastically reducing plant growth and creating widespread famine.
Next: New Research Finds Climate Models Unable to Reproduce Ocean Surface Temperatures